manganese "manganese deposits" "manganese minerals" "manganese ores" "Mn deposits"
Manganese
Manganese is the ninth or tenth most abundant element in the Earth’s crust . Most of its industrial use is in steel making with a much lesser amount going into the production of batteries. It is very similar to iron in its chemical properties. For example both are commonly found in +2 and +3 valences with high spin states for the 3d electrons and with similar ionic radii. Mn2+ is 0.83 and Fe2+ is 0.78 Å, while the +3 ions are 0.70 and 0.65 Å. Accordingly, Mn is commonly found substituted in small amounts in Fe minerals. Mn, however, also has access to a higher valence state, +4, which gives rise to a plethora of complex Mn oxide minerals that do not have Fe counterparts. On the other hand, Mn sulfides are quite rare compared to their Fe cousins. The net result is a partitioning of Mn from Fe in geochemical systems towards areas of higher oxidation potential.
Manganese ores can be carbonates, usually rhodochrosite but sometimes kutnahorite, or various silicates and oxides, the most common being braunite, bixbyite, and hausmannite [see table of common minerals]. The carbonates carry useful isotope signals for C and O [see stable isotopes of Mn deposits].
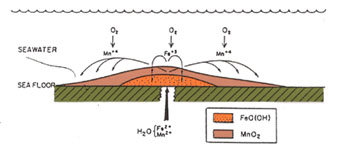
On a purely descriptive basis, Mn ores can be classed as sediment-hosted, volcanic-hosted, or karst-hosted. Chemical distinctions among these types include:
- much higher SiO2 in volcanic rock-hosted deposits, which likely reflects a more oceanic setting with important contributions from pelagic radiolaria and diatoms
- higher P2O5 in sediment-hosted deposits, which may relate to upwelling
- strong enrichment of karstic deposits in Ba and Pb, enabled by the open tunnels in the structure of cryptomelane-group minerals
[see tables of major element and trace element average compositions]
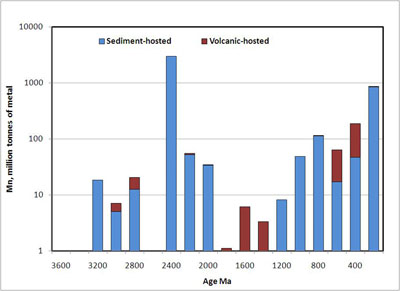
Mn ore deposits are not evenly distributed in geologic time. Instead they cluster in three groups, one in the Paleoproterozoic, the second in the Neoproterozoic and the third in the Cenozoic. There is also a striking disappearance of sediment-hosted deposits from 1800 to 1100 Ma: