Manganese ore deposits carry isotopic signals for carbonate C and O, for sulfide S, and for Fe. Little is known about Fe isotopes in land-based Mn deposits, although there is some data from deep-sea nodules and crusts, but there is considerable data on C-O isotopes and some on S.
C isotopes
Carbon isotopes reflect the source of carbon for the carbonate component of the ore. Many workers report a correlation between the grade of Mn in primary carbonate deposits and the depletion of C in 13C. This pattern indicates that oxidation
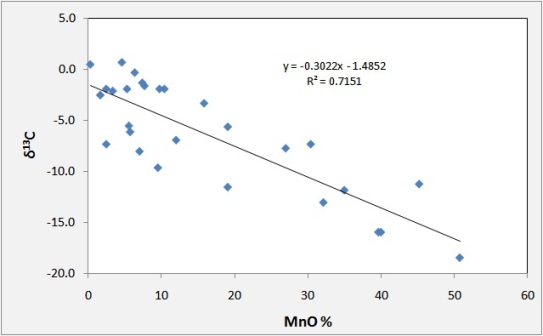
Typical negative relationship between Mn content and C isotopes
of organic matter to CO2 was an integral part of the formation of MnCO3 ores:
2MnO2 + CH2O + HCO3-a 2MnCO3 + H2O + OH-
In this model, about half of the C in the ore would come from organic matter, half from seawater and the maximum depletion of the C in the heavy isotope would be to about -14 permil, assuming an organic C source at -25 to -30 permil.
Of the 31 deposits listed with carbon isotope data, 14 have matching Mn and C data. Of these, 7 show the pattern illustrated for Usa and many of the others have such small data sets that a correlation could be present.
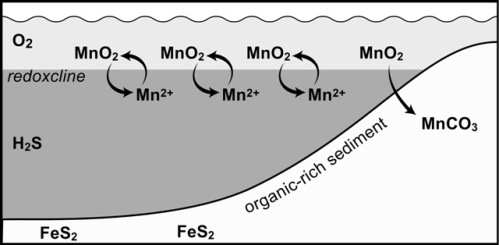
These and most other large Mn deposits likely formed around the margins of euxinic basins (those with free H2S in deep water). Such basins -- exemplified by the modern Black Sea -- have a thin zone of oxygen-containing water overlying a large reservoir of oxygen-free, sulfidic water. Elements like Mn that are soluble under reducing, sulfidic conditions but insoluble under oxidizing conditions precipitate along the interface between these water masses. Generally the Mn solids simply sink back into the sulfidic water and redissolve, but along basin margins, where the transition impinges on the bottom, Mn oxide accumulates in the bottom sediment where it reacts with organic matter to form Mn carbonate (Force and Cannon,1988; Okita, 1987; Okita et al. 1988; Maynard et al., 1990; Okita 1992, and Okita and Shanks, 1992).
Sulfur Isotopes
Sulfur isotopes in this model would be expected to be relatively heavy, about the same as contemporary seawater. At the same time that the manganese is oxidizing the organic matter, it also attacks any iron sulfide in the sediment (Aller and Rude, 1988; Schippers and Jørgensen, 2001):
FeS + 4.5MnO2 + 4H2O = FeOOH + 4.5Mn2+ + SO42- + 7OH-
From this reaction, the deposit should be very low in sulfur, as is observed, and the pyrite that does form should be heavy isotopically, based on the requirement that any pyrite that forms be relatively late, forming after all of the manganese oxide has reacted. Therefore, the degree of contact with the overlying seawater reservoir of sulfate sulfur will be limited, sulfate reduction will go to completion, and the small amount of sulfide that does form will be isotopically close to its parent sulfate compared to normal pyrite in black shale.
Note in the next figure, from the Molango deposit, the convergence of S isotope values on +5-10 permil, far heavier than normal pyritic black shale, which averages -31 permil for the underlying strata at Molango.
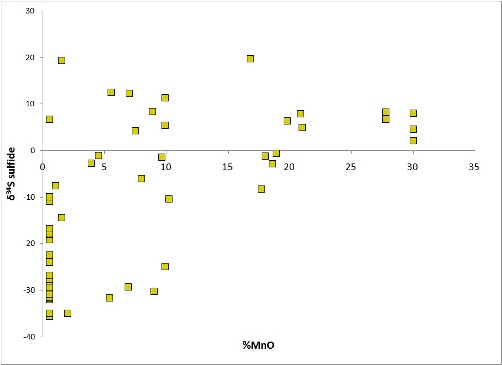
A departure from this pattern for S isotopes is seen in Neoproterozoic strata, which are characterized by super-heavy S values. This phenomenon is not restricted to Mn deposits, however, and appears to be a response to a low-sulfur ocean that could easily have most of its sulfate removed over geologically short time spans (Liu et al., 2006).
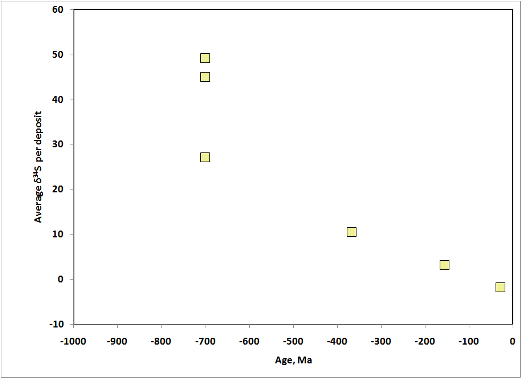
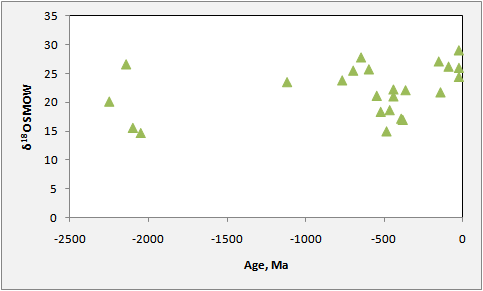
Oxygen Isotopes
Rhodochrosite exchanges oxygen readily with its environment. Therefore O isotopes of Mn deposits reflect post-depositional events more than does C. One would therefore expect that, on average, older deposits would have lighter oxygen than younger ones. As shown below, this is true for the Phanerozoic, but the Proterozoic has no time trend. Why this should be true is not clear.