Corrosion Home
Overview
The response of plumbing components in premise and distribution systems to changes in water treatment is strongly dependent on the mineralogy of the corrosion scales attached to various parts of the system. For lead (Pb) compounds, of which there have been many reported, understanding dissolution-precipitation behavior is key to developing best practices of distribution system management. Knowledge of the crystal form and the degree of crystalinity of these compounds is a necessary contribution to models of Pb behavior and understanding of Pb control via passivation. Accordingly it is critical to develop an understanding of the chemical processes at work involving Pb components in distribution systems.
The dominant Pb minerals found in distribution systems are simple oxides and carbonates (see Table of minerals). Phosphates are also found, and are increasingly relevant as systems turn to orthophosphate dosing to stabilize Pb scales. This array of minerals differs widely in solubility in water depending on the presence of H+, HCO3-, PO43-, SO42-, and the total charge in the solution. Because Pb can occupy three oxidation states (Pb0, Pb2+, and Pb4+), the oxidation level of the water is also critical. (See Oxidation-Reduction).
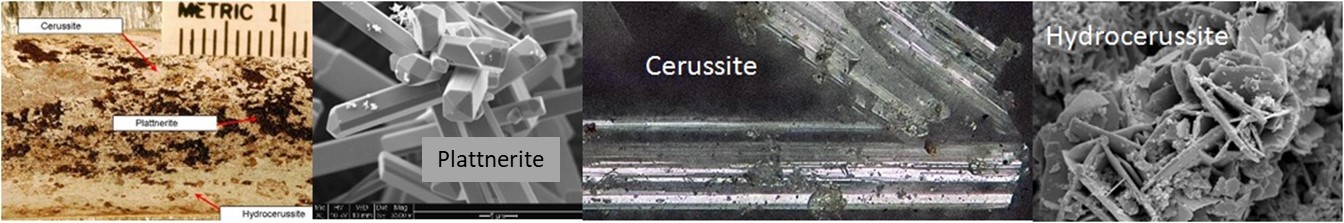
Metalic Pb itself reacts readily with water to produce soluble products, and it is only the formation of protective scales that brings the concentration of Pb in lead piping down into reasonable ranges from a health perspective. Probably the most common protective scales under water distribution system conditions are the two lead carbonates: cerussite and hydrocerussite. However, even for these minerals equilibrium solubility values are still above the Lead and Copper Rule action limit (see Table of Comparisons).
In practice, a well-developed lead carbonate scale does provide protection, because only a portion of the water in the system is in contact with the lead service lines or Pb-containing plumbing materials. Normally, utilities with stable lead carbonate scales will be able to keep lead levels at the tap below the action level with proper system maintenance (unidirectional flushing; low organic carbon; stable pH and alkalinty; stable, high chlorine residual). When debris accumulates in a distribution system or when organic carbon in the water is high, microbial activity in the scale increases, for example denitrification or sulfate reduction, both of which increase corrosion rates. Moreover, if pH, alkalinity, or oxidation state of the water is allowed to vary substantially, scales adapted to one set of conditions become unstable when these conditions change. If water quality fluctuates, the scales are constantly being disrupted (see Flint MI example).
Selected References on Lead
AwwaRF 1990. Lead Control Strategies. Awwa Research Foundation and American Water Works Association. Denver, CO
Cantor, A.F., Denig-Chakroff, D., Vela, R.R., Oleinik, M.G., and Lynch, D.L. 2000. Use of polyphosphate in corrosion control: Jour. AWWA, 92: 96-102.
Edwards, M, and A. Dudi. 2004. Role of chlorine and chloramines in corrosion of lead-bearing plumbing materials: Jour. AWWA, 96: 69-81.
Edwards, M. and McNeil, L.S. 2002. Effect of phosphate inhibitors on lead release from pipes: Jour. AWWA, 96: 69-81.
Hopwood, J. D., R. J.Davey, M. O. Jones, R. G. Pritchard, P. T. Cardew, and A. Booth. 2002, Development of chloropyromorphite coatings for lead water pipes: Journal of Materials Chemistry, v. 12, p. 1717-1723.
Lytle, D.A. and Schock, M.R. 2005. Formation of Pb(IV) oxides: Jour. AWWA, 97: 102-114.
Schock, M. R., and J.A. Clement. 1998. “Lead and copper control with non-zinc orthophosphate”. Journal New England Water Works Association, v. 112, p. 20-42.
Schock, M.R., and R. Giani. 2004. “Oxidant/disinfectant chemistry and impacts on lead corrosion”. Proc. American Water Works Association, WQTC. American Water Works Association. Denver, CO.
Schock, M.R., I. Wagner, and R.J. Oliphant. 1996. “Corrosion and solubility of lead in drinking water”. Chapter 4 in Internal Corrosion of Water Distribution Systems. American Water Works Association Research Foundation/ DVGW Forschungsstelle, Denver CO.
Schock, M.R., S.M. Harmon, J. Swertfeger, and R. Lohmann. 2001. “Tetravalent lead: a hitherto unrecognized control of tap water lead contamination.” Proc. American Water Works Association, WQTC. American Water Works Association. Denver, CO.