In contrast to iron scales from cast iron water mains, scales developed on household galvanized iron carry a substantial load of heavy metals, especially lead (Pb). Therefore particles detached from these scales can be a significant source of Pb measured at the tap, as shown in the table below for a set of galvanized interior pipes from homes with Pb service lines.
Chemistry by ICP of iron scales from galvanized premise plumbing - Utility 1 (bulk scale) |
|||||||||||
| Element | Ca |
Fe |
Mn |
P |
S |
Cd |
Cr |
Cu |
Pb |
V |
Zn |
| Sample | % |
% |
% |
% |
% |
mg/kg |
mg/kg |
mg/kg |
mg/kg |
mg/kg |
mg/kg |
01_PFg11 |
0.17 | 52.6 | 0.46 | 0.13 | 0.70 | 51 | 67 | 2799 | 7700 | 102 | 10285 |
01_PFg13 |
0.56 | 36.4 | 0.80 | 0.38 | 0.34 | 24 | 53 | 4272 | 1064 | 216 | 5800 |
01_PFg14 |
2.07 | 41.8 | 0.27 | 0.67 | 0.49 | 75 | 34 | 2980 | 3760 | 212 | 52990 |
01_PFg15 |
0.58 | 34.7 | 3.57 | 0.56 | 0.29 | 55 | 47 | 6653 | 20190 | 574 | 5230 |
Looking Deeper
Corrosion of iron, whether cast iron or galvanized iron, is generally non-uniform. Scales develop not as a coating on the pipe interior, but as discrete mounds, called tubercles. Does this non-uniform corrosion affect the behavior of Pb?
Corrosion scales on galvanized iron: utility 1 -- the "discovery" sample, 01_PFg11
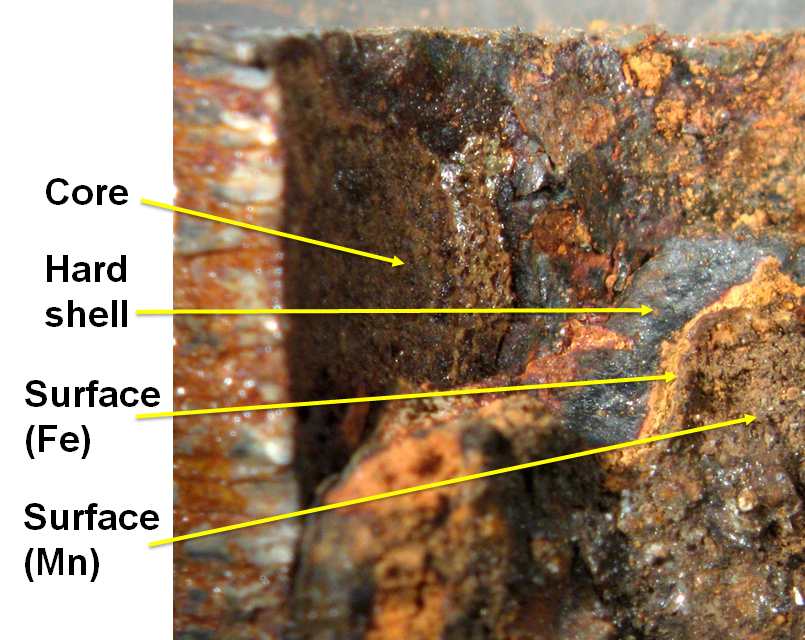
Utility 1 experienced seemingly random occurrences of high total lead in 1st draw tap samples from homes with lead service lines. Scientists at the water authority became suspicious of those homes with galvanized instead of copper or plastic internal plumbing. A sampling program was begun to test the lead concentration in corrosion scales in galvanized pipes downstream of lead services (McFadden et al 2011). This was the intial sample tested. SEM observations showed spots of high Pb. ICP measurements on scraped material (table above) confirmed strkingly elevated lead. Subsequent samples also showed extremely high lead.
How is the Pb held in these scales? This and other scale samples from this utility show considerable morphological variation within a single sample. Is the Pb content uniform or does Pb favor a particular mineralogy or texture? The characteristic structure of iron scales has been described in detail in work by Sarin, Snoeyink and their associates (eg. Sarin et al 2001, 2003; Gerke et al. 2008). Briefly, there are three principal zones, from interior to exterior:
1. Soft core, orange-red -- dominantly goethite;
1a. Veins in the core, dark gray to black -- dominantly magnetite (not seen in this photo)
2. Hard shell layer, black -- magnetite
3. Thin surface layer, tan --3a. amorphous (Fe Layer in photo); 3b. often with high Mn (Mn Layer in photo)
The averages for selected elements of concern (next table) show that the hard shell layer contains lower levels of all variables presented. Pb was too high to be measured by the analytical technique for most samples, but it can be seen that Mn, S, and V are all highest in the surface layer. Because this is the layer most susceptible to chemical or water flow disturbance, it is likely to contribute iron
| 4 pipe samples | Cd | Mn | Pb | S | V |
| Surface layer | 66200 | >5000 | 16800 | 1279 | |
| Hard shell | 2220 | 3829 | 2970 | 137 | |
| Core | 26800 | >5000 | 4980 | 584 | |
| Whole scale | 69 | 10300 | >5000 | 3230 | 612 |
particles with attached heavy metals at levels even higher than would be predicted from the bulk scale analyses.
References
Gerke, T. L., Maynard, J. B., Schock, M. R., & Lytle, D. L. (2008). Physiochemical characterization of five iron tubercles from a single drinking water distribution system: possible new insights on their formation and growth. Corrosion Science, 50(7), 2030-2039.
McFadden, M., Giani, R., Kwan, P., & Reiber, S. H. (2011). Contributions to drinking water lead from galvanized iron corrosion scales. Journal‐American Water Works Association, 103(4), 76-89.
Sarin, P., Snoeyink, V. L., Bebee, J., Kriven, W. M., & Clement, J. A. (2001). Physico-chemical characteristics of corrosion scales in old iron pipes. Water Research, 35(12), 2961-2969.
Sarin, P., Clement, J. A., Snoeyink, V. L., & Kriven, W. M. (2003). Iron Release from corroded, unlined cast‐iron pipe. Journal‐American Water Works Association, 95(11), 85-96.