Corrosion of iron, whether cast iron or galvanized iron, is generally non-uniform. Scales develop not as a coating on the pipe interior, but as discrete mounds, called tubercles, that have distinct morphological components with different chemistries and mineralogies. Scales on galvanized iron show the same morphological varieties as on cast iron, from interior to exterior:
1. Soft core, orange-red -- dominantly goethite (FeOOH) or sometimes lepidocrocite
1a.. Veins in the core, dark gray to black -- dominantly magnetite
2. Hard shell layer, black -- magnetite
3. Thin surface layer, tan -- amorphous, often with high Mn
Three pipes from Utility 01 illustrate tubercles on galvanized pipe:
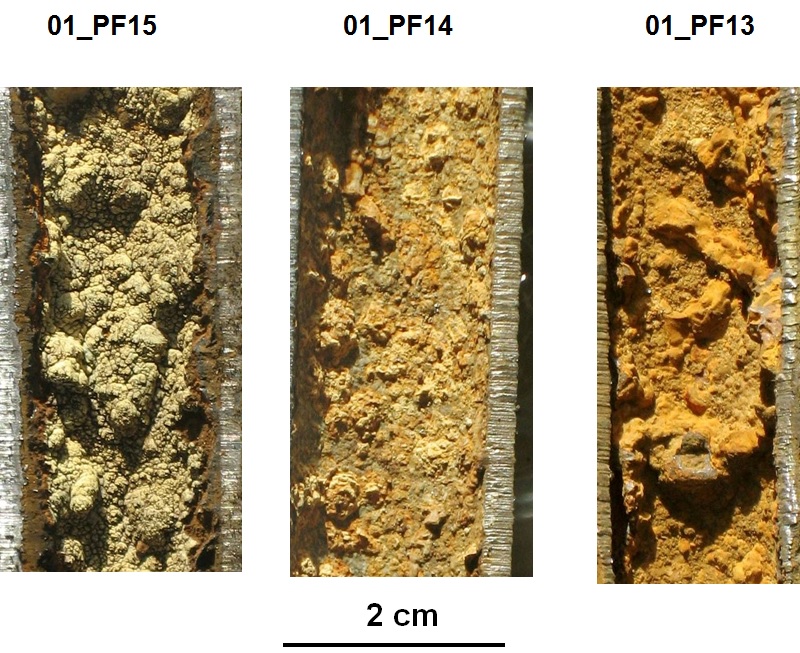 |
|---|
Examples of corrosion scales on galvanized iron: utility 1 - chemistry of morphological varieties
 |
Note highest values for metals in surface layer; lowest in hard shell layer. Magnetite has relatively low affinity for metals compared to goethite. |
|---|
 |
Very high Mn associated with very high Pb and higher Cd. S is highest in core again, and is highest among the 3 core samples. Biological? Concentration in deeper layers suggest sulfate reducing bacteria.
|
|---|
 |
Highest Mn among the 3 samples. Pb generally too high for quantification by method used |
|---|