Lead to brass connections
When two metals are connected, accelerated corrosion can set in with one metal essentially attacking the other, a process termed galvanic corrosion. In the consumer's portion of a drinking water distribution system, there are several likely places for lead-to-brass couplings that can experience this accelerated reaction.
A house with a lead service line may have 6 lead-to-brass connections, as  shown in the diagram to the right. Not only is pipe corrosion accelerated at these junctions, but corrosion products build up on the inner surface of the pipe, creating an obstruction that will generate turbulence in the flow and increase the chance of detaching particles that could transfer lead-containing solids to the cusotmer's tap. This kind of release is dangerous because a large amount of lead can move in a short time, it can happen at anytime during the day and so cannot be prevented by flushing the tap, and the lead carried by these particles is likely not included in water sampling to assess lead exposure.
shown in the diagram to the right. Not only is pipe corrosion accelerated at these junctions, but corrosion products build up on the inner surface of the pipe, creating an obstruction that will generate turbulence in the flow and increase the chance of detaching particles that could transfer lead-containing solids to the cusotmer's tap. This kind of release is dangerous because a large amount of lead can move in a short time, it can happen at anytime during the day and so cannot be prevented by flushing the tap, and the lead carried by these particles is likely not included in water sampling to assess lead exposure.
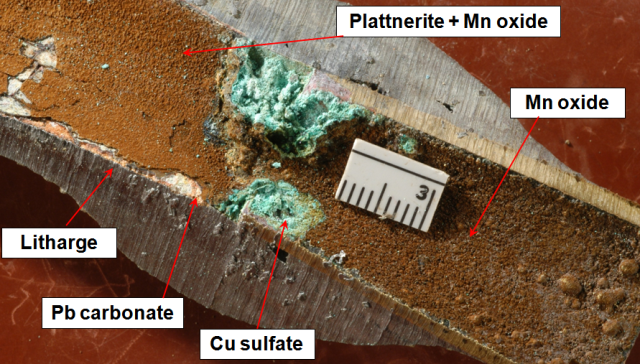
As shown below, the build-up of corrosion products at such junctions can be severe. [click to enlarge]. Note Zn-depleted brass at contact.
See DeSantis and others (2009) for mineralogy of these corrosion scales
Schematic view of Pb-(brass)-copper galvanic reactions

For more information see Welter and others (2013)