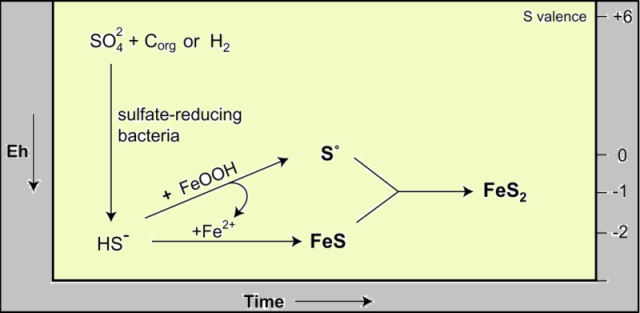
The conversion of sulfate (SO42-), with an oxidation state for sulfur of +6, to native sulfur (S0) or to sulfide (S2-) is a very slow reaction at ordinary temperatures and requires the intervention of sulfate-reducing bacteria. The organisms use the oxygen in the sulfate to digest organic matter in their environment, in the process releasing CO2 and H2S.
This reaction is widespread in soils, in lakes, and in marine sediments. The H2S reacts quickly with iron to produce FeS, which has an intense black color.

FeS-bearing mud in a constructed wetland receiving sulfur-rich acid mine drainage. Bacteria use the organic matter from the cattail leaves to oxidize the sulfate in the water to sulfide, which then percipitates the iron. A tracer of this bacterial activity is the isotopic composition of the sulfur (see Flege and others 2012). In these wetlands, there is a spread of isotope values between the water and the precipitated reduced S of 19 to 35 permil. Only bacterial activity produces these kinds of fractionations.
Sulfur isotopes have rarely been reported from distribution system scales. An exception is Lytle and others (2005) who studied iron scales from water mains from two systems. One system had a spread in sulfur isotopes of 14 permil between sulfate and sulfide, the other was 40 permil. Note the range is essentially identical to the wetlands data, confirming that sulfur reducing bacteria are flourishing in these corrosion scales. Instead of FeS, the dominant product in pipe scales seems to be native sulfur (aka zero-valent S).

Sulfur in iron corrosion scale from unlined cast iron water main. The native S is original to the scale; the gypsum (calcium sulfate) forms by oxidation of the reduced S on exposure to air.
Proposed sequence for production of S0 in corrosion scales:
References to sulfate reduction
Lytle,